
miR-124/PTBP1/PKM, a new axis that fuels pulmonary hypertension
Pulmonary hypertension (PH) (1) is a recalcitrant disease that is increasingly recognized as a critical contributor of cardiovascular mortality worldwide. While considerable progress has been made in the understanding of pathophysiology of PH, significant knowledge gaps still remain. At the same time, our therapeutic repertoire continues to be painfully limited despite additions of several novel classes of PH medications (1,2). These oft-termed advanced therapies [represented by prostaglandin analogues, endothelin-receptor antagonists, and soluble guanylyl cyclase agonists] often find their use hindered by cost as well as by suboptimal therapeutic response. The latter arises partially owing to their collective mechanistic dependence on vasodilation, which overlaps with older-generation agents such as calcium-channel blockers and phosphodiesterase 4 (PDE4) inhibitors. While further dissecting the same set of fundamental pathways may yield incremental benefits, we may need to take a drastically different angle of approach in order to discover drugs with truly ground-breaking efficacy.
In their study published in 12/2017 issue of Circulation, Zhang et al. provided an elegant example of exploring alternative therapeutic approaches with transformative thinking and techniques (3). Over the years, the field of PH research focused on a few signaling pathways involved in vasoconstriction, a process that primarily takes place within the pulmonary smooth muscle layer with regulatory input from the endothelium. In the process, we often overlooked the third histological layer, tunica adventitia, which also contributes to the pathogenesis of PH by way of perivascular inflammation and fibroproliferative remodeling. Fibroblasts are key mediators of the above processes (4,5). Having previously observed that PH-derived fibroblasts displayed profound proliferative, pro-migratory and pro-inflammatory phenotype (6), Zhang et al. built upon the intriguing premise that these fibroblasts undergo metabolic transformation similar to cancer cells (7,8), sacrificing the ATP-efficient oxidative phosphorylation of the mitochondrial citric acid cycle (TCA cycle) cycle and opting instead for aerobic glycolysis that allows generation of metabolic intermediates to be used as cellular building blocks. This phenomenon of metabolic plasticity would transform fibroblasts in PH patients [pulmonary hypertension fibroblast (PH-fibs)] at the very metabolic level, re-routing them to an uninhibited, pro-proliferative cell fate.
Using previously validated techniques, Zhang et al. isolated and cultured fibroblasts from bovine PH models as well as human tissue samples from PH patients and healthy controls. They focused on pyruvate kinase (PK), the enzyme catalyzing the final step of glycolysis, and its two functionally distinct splicing isoforms, pyruvate kinase muscle 1 (PKM1) and pyruvate kinase muscle 2 (PKM2). The group noted that PKM2/PKM1 ratio was significantly increased in PH fibroblasts versus that of the controls. This in vitro finding, previously reported in certain subtypes of cancer, was readily reversed with knockdown of PKM2 with isoform-specific siRNA, which interestingly reversed PH-fibs’ abnormal metabolic signature and also blunted their proliferation in vitro. Polypyrimidine tract binding protein 1 (PTBP1), an alternative splicing factor previously reported to promote PKM2 generation in certain cancer cells (9,10), was found to play a key role in regulating PKM2/PKM1 (6), as siRNA knockdown of PTBP1 decreased the abnormally high PKM2/PKM1 ratio in PH-fibs at both mRNA and protein level. This normalization of PKM2/PKM1 ratio led to restoration of wild-type metabolomics profile in PH-fibs, a finding also seen with treatment by small-molecule modulators/inhibitors of PKM2, TTEP-46 and shikonin.
Interestingly, similar metabolic rescue was reproduced when PH-fibs were treated with a mimic of miR-124, an endogenous microRNA of which PTBP1 is a direct target. miR-124 had previously been shown to be pro-apoptotic in glioblastoma (11) and to promote neuronal differentiation via alternative splicing (12), and its down-regulation was linked with tumor invasiveness in various cancers including gastric, bladder and breast cancer (13-15). Additionally, miR-124 was shown to inhibit proliferation in pulmonary artery smooth muscle cells (16) and endothelial cells (17). Using an array of assays including RT-PCR of native transcripts, PKM2 splicing reporter plasmid, western blot, as well as redox assays including respiratory control ratio and mitochondrial superoxide production, Zhang et al. demonstrated that miR124 exerts direct control over PTBP expression, which in turn regulates PKM2/PKM1 ratio and mitochondrial metabolism. It is the first time that a microRNA is established as a central mediator of metabolic homeostasis in pulmonary fibroblasts and thereby the process of adventitial fibrosis and proliferation (see Figure 1 for a graphic illustration of the pathway).
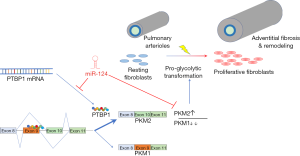
In this study, the role of non-coding RNA is brought into sharp focus. MicroRNAs (or miRNA) are a well-recognized set of noncoding RNAs ~22 nucleotide in length that regulate a wide range of gene expressions through binding to 3’ untranslated region (3’UTR) of target mRNAs, leading to transcript degradation or translational inhibition. Since its discovery in 1993, miRNA has been broadly implicated in many disease pathogenesis including carcinogenesis, heart failure progression and remodeling (18,19), vascular remodeling (20), and even PH (21-23). However, it was speculative at best on how such knowledge might translate into usable clinical tools. Identification of miR-124 as a repressed target with functional importance in the pathogenesis of PH makes it an ideal candidate as a replacement therapy. Indeed, one published report may give a proof-of-concept glimpse, where a miR-124 mimic co-delivered with a Bcl-2 inhibitor in a micelle-based vehicle demonstrated pro-apoptotic effects in breast cancer (24). On a broader perspective, it could join a growing stream of novel oligonucleotide-based therapies on the market or undergoing clinic trials. Antisense oligonucleotides (ASOs) are a class of synthetic siRNA/miRNA mimetics using a variable of natural and chemically modified backbones. Remarkably, they are able to achieve gene regulation in a variety of ways, including mRNA transcript degradation [RNAse H activation or RNA-induced silencing complex (RISC) recruitment], translational inhibition (ribosome assembly disruption or steric hindrance), splicing alteration, and miRNA sequestration (25,26). In 2013, mipomersen was approved by Food and Drug Administration (FDA) for treatment of homozygous familial hypercholesterolemia, which targets apolipoprotein B100 (Apo-B 100) and directs its mRNA degradation via RNase-H activation. PTBP1, overexpressed in PH-fibs, could similarly serve as a valuable therapeutic target, as its in vitro knockdown by siRNA led to mitochondrial metabolic normalization. More recently, two other drugs received accelerated approval from the FDA: eteplirsen for treatment of Duchenne muscular dystrophy (DMD) via exon skipping, and nusinersen for treatment of spinal muscular dystrophy (SMA) via exon retention. The success of these two agents brings into consideration whether a similar approach could be viable in modulating relative expressions of PKM1 & PKM2, which differ only by the inclusion choice of a single exon (exon 9 vs. 10, respectively). It is conceivable that high-throughput screening can identify ASOs with the right sequence and chemical modifications to promote retention of exon 9 over exon 10 (action in opposite to PTBP), thus restoring PKM1/PKM2 ratio in PH-fibs and slowing/reversing adventitial fibrosis and remodeling in the pulmonary vasculature.
This article by Zhang et al. is significant in several unique ways. First, they zoomed in on a separate cell population (fibroblasts) and were able to replicate their findings in both cells from bovine PH models as well as actual human tissues at the time of lung transplant, thus affirming generalizability of their findings. Second, they viewed the field through a metabolic lens, identifying a key regulatory step in glycolysis (miR-124-PTBP1-PK axis) that disproportionally impacts a cell’s metabolomics profile during PH development. Third and most importantly, the team demonstrated that single-agent intervention at multiple steps of this pathway each led to measurable corrective effects on mitochondrial and cellular bioenergetics. While the results using PKM2 inhibitor/modulators TEPP-46 and shikonin and using histone deacetylases (HDAC) inhibitors offered prospective for traditional small-molecule-based drug strategy, it is the impressive effects of siRNA and miRNA mimetics that may finally usher RNA-based agents onto the stage of PH therapy.
As much as this study was thought-provoking, it had a number of limitations. For instance, non-bioenergetic aspects of mitochondrial function (e.g., Caspase-mediated apoptosis pathway) may be impacted by alterations in PKM2/PKM1 ratio and warrant further study. Second, while changes in the fibroblast proliferative rate in vitro was measured as the main physiologic readout, in vivo aspects of adventitial remodeling in PH (e.g., changes in extracellular matrix or composition; macrophage recruitment/activation) were not examined. The authors did present in the supplemental data (albeit preliminary) that shikonin treatment of mice subjected to hypoxic insult led to normalization of right ventricular (RV) systolic pressure as well as reduction in proliferative, pro-inflammatory, and pro-remodeling markers on lung tissue histology. It would be very interesting to assay for these physiologic effects with direct genetic modulation of PKM2/PKM1 expression, first by generating conditional knockout or transgenic mouse models and eventually using lung-specific ASO and siRNA-delivery vehicles (e.g., nebulization) as explorative therapy.
In summary, Zhang et al. presented the miR-124/PTBP1/PKM axis as a key pro-glycolytic switch that promotes PH fibroblast proliferation via aerobic glycolysis decoupling and cellular metabolic transformation. The study also provided riveting clues that this pathway plays a central role in PH pathogenesis via adventitial remodeling and could be appropriated at multiple steps, most intriguingly at the level of mRNA expression and alternative splicing with ASO-based therapy. The collective findings from this study point to a paradigm shift in the fundamental understanding of PH and intriguing directions towards unlocking brand-new therapeutic options.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shengguang Ding (The Second Affiliated Hospital of Nantong University, Nantong, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2018.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573-619. [Crossref] [PubMed]
- Ghataorhe P, Rhodes CJ, Harbaum L, et al. Pulmonary arterial hypertension - progress in understanding the disease and prioritizing strategies for drug development. J Intern Med 2017;282:129-41. [Crossref] [PubMed]
- Zhang H, Wang D, Li M, et al. Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension Is Regulated Through a MicroRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/Pyruvate Kinase Muscle Axis. Circulation 2017;136:2468-85. [PubMed]
- Sartore S, Chiavegato A, Faggin E, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res 2001;89:1111-21. [Crossref] [PubMed]
- Stenmark KR, Yeager ME, El Kasmi KC, et al. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol 2013;75:23-47. [Crossref] [PubMed]
- Wang D, Zhang H, Li M, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res 2014;114:67-78. [Crossref] [PubMed]
- Cottrill KA, Chan SY. Metabolic dysfunction in pulmonary hypertension: the expanding relevance of the Warburg effect. Eur J Clin Invest 2013;43:855-65. [Crossref] [PubMed]
- Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res 2014;115:148-64. [Crossref] [PubMed]
- Clower CV, Chatterjee D, Wang Z, et al. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci U S A 2010;107:1894-9. [Crossref] [PubMed]
- Chen M, David CJ, Manley JL. Concentration-dependent control of pyruvate kinase M mutually exclusive splicing by hnRNP proteins. Nat Struct Mol Biol 2012;19:346-54. [Crossref] [PubMed]
- Zhao WH, Wu SQ, Zhang YD. Downregulation of miR-124 promotes the growth and invasiveness of glioblastoma cells involving upregulation of PPP1R13L. Int J Mol Med 2013;32:101-7. [Crossref] [PubMed]
- Makeyev EV, Zhang J, Carrasco MA, et al. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 2007;27:435-48. [Crossref] [PubMed]
- Xia J, Wu Z, Yu C, et al. miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J Pathol 2012;227:470-80. [Crossref] [PubMed]
- Xu X, Li S, Lin Y, et al. MicroRNA-124-3p inhibits cell migration and invasion in bladder cancer cells by targeting ROCK1. J Transl Med 2013;11:276. [Crossref] [PubMed]
- Han ZB, Yang Z, Chi Y, et al. MicroRNA-124 suppresses breast cancer cell growth and motility by targeting CD151. Cell Physiol Biochem 2013;31:823-32. [Crossref] [PubMed]
- Kang K, Peng X, Zhang X, et al. MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J Biol Chem 2013;288:25414-27. [Crossref] [PubMed]
- Caruso P, Dunmore BJ, Schlosser K, et al. Identification of MicroRNA-124 as a Major Regulator of Enhanced Endothelial Cell Glycolysis in Pulmonary Arterial Hypertension via PTBP1 (Polypyrimidine Tract Binding Protein) and Pyruvate Kinase M2. Circulation 2017;136:2451-67. [PubMed]
- Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol 2018;15:83-96. [Crossref] [PubMed]
- Shah R, Ziegler O, Yeri A, et al. MicroRNAs Associated With Reverse Left Ventricular Remodeling in Humans Identify Pathways of Heart Failure Progression. Circ Heart Fail 2018;11:e004278 [Crossref] [PubMed]
- Deng L, Bradshaw AC, Baker AH. Role of noncoding RNA in vascular remodelling. Curr Opin Lipidol 2016;27:439-48. [Crossref] [PubMed]
- Boucherat O, Potus F, Bonnet S. microRNA and Pulmonary Hypertension. Adv Exp Med Biol 2015;888:237-52. [Crossref] [PubMed]
- Courboulin A, Paulin R, Giguere NJ, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011;208:535-48. [Crossref] [PubMed]
- White K, Lu Y, Annis S, et al. Genetic and hypoxic alterations of the microRNA-210-ISCU1/2 axis promote iron-sulfur deficiency and pulmonary hypertension. EMBO Mol Med 2015;7:695-713. [Crossref] [PubMed]
- Zhang N, Huang Y, Wu F, et al. Codelivery of a miR-124 Mimic and Obatoclax by Cholesterol-Penetratin Micelles Simultaneously Induces Apoptosis and Inhibits Autophagic Flux in Breast Cancer in Vitro and in Vivo. Mol Pharm 2016;13:2466-83. [Crossref] [PubMed]
- Martinovich KM, Shaw NC, Kicic A, et al. The potential of antisense oligonucleotide therapies for inherited childhood lung diseases. Mol Cell Pediatr 2018;5:3. [Crossref] [PubMed]
- Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res 2018;46:1584-600. [Crossref] [PubMed]
Cite this article as: Yu D, Kim GH. miR-124/PTBP1/PKM, a new axis that fuels pulmonary hypertension. Non-coding RNA Investig 2018;2:21.


