
Circling the PINK away: suppression of autophagy by a novel ncRNA-mediated mechanism
Pervasive transcription of the human genome is responsible for the generation of thousands of non-coding RNA molecules (ncRNAs) with regulatory function. These ncRNAs can be generated either by the transcription of specific genomic loci or by the processing of already synthesized RNAs. The regulatory roles of these molecular species have special importance in non-proliferative organs such as the heart, where a delicate balance of molecular interactions is needed to ensure its biogenesis and function.
Among ncRNAs, circular RNAs (circRNAs) are a recently discovered family of ncRNA molecules generated by non-canonical splicing events (1). The back-splicing phenomenon involves the production of circRNA molecules generated by covalent bonds between the 5' and 3' ends of a splicing product. Firstly, described as splicing by-products, they were lately involved in regulatory events essential for cellular homeostasis and function (2). Among other mechanisms, circRNAs appeared to preferentially function as molecular scavengers of proteins or RNAs (mainly ncRNAs), constituting a “buffering” mechanism used by cells for a delicate control of the levels of specific biomolecules. The molecular rules governing the scavenging or “sponging” activity of circRNAs appeared to be clear for RNAs, being controlled by the sequence complementarity between the circRNAs and the target RNA (3). However, the molecular mechanism and selectivity of protein sponging by circRNAs is still not well understood (4).
Cardiology is one of the research areas historically more interested in the physiological role of ncRNAs. Since the discovery of ncRNAs, the role of many of them in cardiovascular development and disease has been described with special focus on microRNAs (miRNAs) (5). Recently, the discovery of RNA-crosstalk mechanisms mediated by direct interactions between several ncRNA species has been also related with cardiovascular diseases (6). In the center of these RNA-interaction networks, circRNAs have an essential role due to their sponging activity of other ncRNAs such as miRNAs (7,8). In some well documented cases, the scavenging activity mediated by circRNAs over miRNAs have a protective effect over the deleterious action exerted by an excess of miRNA expression. This is the case of the heart-related circRNA (HRCR), which is able to sponge miR-223, preventing its action and protecting the heart from hypertrophic cardiomyopathy and functional failure (9).
However, the role of circRNAs as essential players in cardiovascular homeostasis and function has been expanded with the characterization of their functional interactions with proteins. In this context, the manuscript by Zhou and coworkers (10) described a novel mechanism of autophagy inhibition involving a circRNA named autophagy-related circRNA (ACR) (Figure 1). Autophagy is a central cellular process that is extremely relevant in the context of many cardiovascular disease, due to its implication in cardiac fibrosis and necrosis after an external myocardial insult. Different types of negative stimuli have been linked with heart failure and disfunction, but among them, the oxidative stress is one of the best characterized. Any imbalance among the different reactive oxygen species (ROS) and the molecular mechanisms used to eliminate their toxic effects would lead to an oxidative stress. In the heart the main source of ROS are the mitochondria, where several important molecular actors have been identified. PTEN inducible kinase 1 (PINK1), has been related with the regulation of oxidative stress, since mutations in the PINK1 gene lead to mitochondrial disfunction, increased sensitivity to ROS and apoptosis in neurodegenerative diseases. Interestingly, PINK1 knock-out mice develop left ventricular dysfunction and evidence of pathological cardiac hypertrophy as early as 2 month of age, consequence of the increased levels of oxidative stress induced by ROS and the impaired mitochondrial function (11). In the heart, PINK1 levels are epigenetically regulated by promoter methylation catalyzed by Dnmt3b DNA methyltransferase. Zhou and coworkers characterized how the ACR circRNA is able to sequester Dnmt3b, inducing a PINK1 promoter demethylation and consequent overexpression of the PINK1 coding transcript. PINK1 downstream targets are very wide, but in the heart is able to phosphorylate the FAM65B protein which is a main regulator of autophagy (10). This time, the regulatory effect of a circRNA is exerted via protein sponging, to control a tripartite axis composed by Dnmt3B, PINK1 and FAM65B proteins (Figure 1). In the presence of ACR circRNA the cardiac cells are protected against autophagy and cell death, even being able to partially restore the necrotic phenotype in animal models. This protective regulatory effect of ACR circRNA is in clear contrast with the already characterized regulatory effect of other circRNA named circ-Foxo3, which is able to interact with the anti-senescent protein ID-1 and the transcription factor E2F1, as well as the anti-stress proteins FAK and HIF1α, leading to a senescent phenotype in cardiac cells (12).
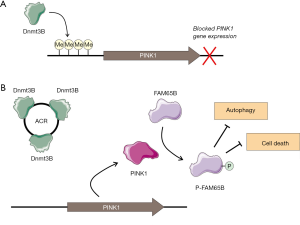
There is still a long road to pave, but ACR circRNA constitutes an open door for exploring new therapeutic options for cardioprotection and cardiac regeneration. As adjuvant strategy, the manipulation of the ACR levels in selected pathologies as myocardial infarction could help to increase the cardiomyocyte regeneration potential and to partially restore necrotic lesions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shengguang Ding (The Second Affiliated Hospital of Nantong University, Nantong, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri.2019.03.05). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol 2014;32:453-61. [Crossref] [PubMed]
- Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013;495:333-8. [Crossref] [PubMed]
- Rong D, Sun H, Li Z, et al. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget 2017;8:73271-81. [Crossref] [PubMed]
- Zang J, Lu D, Xu A. The interaction of circRNAs and RNA binding proteins: An important part of circRNA maintenance and function. J Neurosci Res 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Huang W, Yu Q, Wang Q, et al. Roles of miRNA in cardiovascular development and dysfunction. Curr Med Chem 2013;20:3613-22. [Crossref] [PubMed]
- Holdt LM, Kohlmaier A, Teupser D. Molecular functions and specific roles of circRNAs in the cardiovascular system. Noncoding RNA Res 2018;3:75-98. [Crossref] [PubMed]
- Elia L, Quintavalle M, Condorelli G. Circular RNAs and heart failure: new players for an old disease. Cardiovasc Res 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Hu X, Chen L, Wu S, et al. Integrative Analysis Reveals Key Circular RNA in Atrial Fibrillation. Front Genet 2019;10:108. [Crossref] [PubMed]
- Wang K, Long B, Liu F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J 2016;37:2602-11. [Crossref] [PubMed]
- Zhou LY, Zhai M, Huang Y, et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/ FAM65B pathway. Cell Death Differ 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Billia F, Hauck L, Konecny F, et al. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci U S A 2011;108:9572-7. [Crossref] [PubMed]
- Du WW, Yang W, Chen Y, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J 2017;38:1402-12. [PubMed]
Cite this article as: Enguita FJ. Circling the PINK away: suppression of autophagy by a novel ncRNA-mediated mechanism. Non-coding RNA Investig 2019;3:15.


