
Exercise prevents cardiovascular dysfunctions in diabetes through miRNAs modulation
Type 2 diabetes mellitus (T2DM) is a widespread condition worldwide, linked with an increased risk of developing some form of heart disease, referred to as “Diabetic Heart Disease” (DHD) (1). In particular, DHD is characterized by a progression of coronary endothelial and vascular dysfunction, and left ventricular dysfunction and remodeling, which impact on cardiac function (2).
Although many approaches to reducing cardiovascular risk in diabetes have been developed, the possibility to manifest heart failure in these patients is still present. Several experimental studies have shown that active lifestyle or increased physical activity is an effective non-pharmacological approach to reduce cardiovascular events and to support the pharmacological therapy of DHD (3,4). In T2DM subjects, regular exercise is able to improve myocardial function with amelioration of endothelial and left ventricular function, but positive effects of exercise on DHD might depend on both exercise intensity and on the moment in which it is started (5-7). However, very often exercise is initiated when cardiovascular diseases are present. Therefore, it is not clear whether early exercise training or high intensity exercise approaches would be more effective in limiting T2DM related cardiovascular complications.
In order to investigate the efficacy and mechanisms of exercise training in preventing cardiovascular dysfunction during the onset and progression of DHD, in a recent issue on Circulation Research, Lew et al. (8), used a diabetic mouse model (db/db) subjected to moderate or high intensity exercise for 8 weeks. At this age this model is characterized by hyperinsulinemia and hyperglycemia, with a typical obese phenotype without apparent cardiac and coronary dysfunction. The authors demonstrated that both moderate and high intensity exercise training prevent the disruption of coronary and cardiac dysfunction if started in an early stage of diabetes (by 8 weeks of age) and continued for 8 weeks. On the contrary, if exercise is initiated in an advanced diabetic phase, after DHD has already become established (i.e., in 16 weeks old mice), only high, and not moderate exercise training is able to mitigate cardiovascular dysfunction (Figure 1). Therefore, this study confirms that exercise is beneficial, but suggests that benefits depend on timing and intensity. The fact that only high intensity exercise is beneficial in the late stages is worrying as physicians may be reluctant to recommend high intensity exercise when cardiac and coronary dysfunction are present. Therefore, once again we can conclude that regular, early in life, moderate exercise is better.
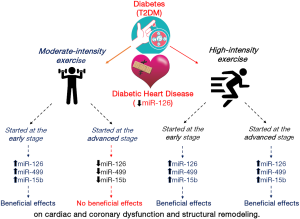
An important aspect of the article by Lew et al. (8) is the role of microRNAs (miRNAs), a class of noncoding RNAs (~22 nucleotides), recognized as main molecular regulators of pathophysiological processes (3). Indeed, it has been reported that the structural and functional myocardial changes related to the progression of diabetes seem to be associated with alterations in the expression of microRNAs (miRNAs). Several preclinical studies have shown that the dysregulation of specific miRNAs is associated with vascular damage in DHD, therefore the modulation of cardiac miRNA expression may have beneficial effects in reducing cardiovascular abnormalities in diabetes (9). Exercise training represents a promising tool to modulate miRNA dysregulation and to reduce diabetes-induced cardiac damage (10,11). Among the miRNAs involved in regulating angiogenesis and vascular integrity in the DHD, miR-126 seems to be one of the most important (12). Thanks to the location of miR-126 in an intron of Egfl7, a gene that is highly expressed in endothelial cells, it represents the most enriched miRNA in endothelial cells (13,14). miR-126, through the repression of sprout-related protein-1 (Spred-1), which is the main negative regulator of the vascular endothelial growth factor (VEGF) signaling, promotes the activation of VEGF pathway. For this reason, the miR-126 represents an important regulator of angiogenesis, endothelial proliferation, differentiation, and survival. Indeed, DHD is characterized by downregulation of VEGF, which is a proangiogenic factor, and by upregulation of Spred-1, which is an antiangiogenic protein.
Besides to show that only high-intensity exercise was effective in late stage, Lew et al. (8) highlighted the role of miR-126 as a potential diagnostic biomarker for the detection of vascular dysfunctions in early diabetes. Real time PCR revealed the circulating and cardiac reduction of miR-126 expression before exercise and its overexpression at the end of the exercise training. Interestingly, miR-126 silencing, using an injection of anti-miR-126, abolished the exercise-mediated protection, while miR-126 upregulation, using an injection of pre-miR-126 mimic, restored the protective effect of moderate exercise training in advanced phase of diabetes. Additionally, as already demonstrated by other authors (15,16), Lew et al. (8) revealed the anti-apoptotic and anti-fibrotic role of miR-499 and miR-15b, respectively. The authors found that both moderate and high-intensity exercise favored the increased of miR-499 and miR-15b expression (Figure 1), while in DHD these miRNAs were downregulated, with significant upregulation of anti-angiogenic Spred-1 (miR-126 target), pro-fibrotic CTGF (miR-15b target) and pro-apoptotic cleaved caspase-3 (miR-499 target).
In the study by Lew et al. (8), to assess structural and functional cardiac and coronary changes in diabetic mice, many methodological approaches were used. Echocardiographic data revealed that 16 weeks old diabetic mice showed systolic and diastolic dysfunctions and structural remodeling of the left ventricle. Both moderate and high intensity exercise were able to prevent these impairments when initiated from 8 weeks of age. Although high intensity exercise was an effective tool to improve systolic and diastolic dysfunctions and structural remodeling of the left ventricle in 16 weeks old diabetic mice, moderate intensity exercise training was not effective. A blood sample was collected to detect hemoglobin A1c (HbA1c), but no significant difference in the level of HbA1c among the exercise DM groups has been shown. Coronary microangiography results indicated that basal coronary perfusion was not significantly impaired at 16 weeks of age in diabetic mice. However, immunohistochemistry (IHC) staining revealed that coronary terminal arteriole density, and both functional and total capillary density were reduced in the diabetic heart at 16 weeks of age. Therefore, the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end-labeling (TUNEL) staining revealed that diabetes induces a progressive increase of cardiomyocyte apoptosis, in particular in 16 weeks old diabetic mice. Both moderate and high intensity exercise were able to reduce cardiomyocyte apoptosis, only when physical activity was initiated before the development of cardiovascular dysfunction.
Therefore, as previously shown in the literature, Lew et al. (8), confirmed the alteration of coronary perfusion which lead to cardiac dysfunction in T2DM. The efficacy of increased physical activity has been shown to be variable depending on exercise intensity and the phase at which training started. The impairment of vascular miRNAs, miR-126, miR-15b, and miR-499 lead to cardiovascular pathological remodeling in DHD. However, for the first time, the authors demonstrated that exercise intensity, and the timing at which the exercise is initiated after early diabetes diagnosis, are key factors for cardiovascular integrity. In addition, they identified an important role of non-coding miRNAs as biomarkers of the development of DHD and as effectors through which exercise is able to mediate its cardioprotection. The detection of plasma miR-126 may help in understanding the early prognosis of DHD and, thus, may be used as a potential biomarker in diabetic patients.
Physical inactivity figures as one of the most important factors of the burden of T2DM, coronary heart disease, breast and colon cancers and premature mortality, killing more than 5 million people every year, besides its social and economic impacts (17,18). Worldwide, 3 in 4 adolescents (aged 11–17 years) and 1 in 4 adults do not currently meet the global recommendations of 150 minutes per week of moderate-intensity physical activity, which is even more challenging now during the COVID-19 pandemic (19,20). From the study of Lew et al. (8) it is clear that in pre-diabetic conditions a moderate early exercise may be beneficial. Therefore, people should, in any case, keep exercising whenever possible to reduce the burning deriving from inactivity.
Acknowledgments
We thank Prof. Claudia Penna for the critical reading of the final manuscript. The authors are supported by University of Torino, Italy.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Tingting Yang (Shanghai University, Institute of Cardiovascular Sciences, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ncri-20-8). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Katare R, Pearson JT, Lew JK, et al. Progressive Decrease in Coronary Vascular Function Associated With Type 2 Diabetic Heart Disease Front Physiol 2018;9:696. [Crossref] [PubMed]
- Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol 2018;17:57. [Crossref] [PubMed]
- Lew JK, Pearson JT, Schwenke DO, et al. Exercise mediated protection of diabetic heart through modulation of microRNA mediated molecular pathways. Cardiovasc Diabetol 2017;16:10. [Crossref] [PubMed]
- Crisafulli A, Pagliaro P, Roberto S, et al. Diabetic Cardiomyopathy and Ischemic Heart Disease: Prevention and Therapy by Exercise and Conditioning. Int J Mol Sci 2020;21:2896. [Crossref] [PubMed]
- Brassard P, Legault S, Garneau C, et al. Normalization of diastolic dysfunction in type 2 diabetics after exercise training. Med Sci Sports Exerc 2007;39:1896-901. [Crossref] [PubMed]
- Hollekim-Strand SM, Bjørgaas MR, Albrektsen G, et al. High-intensity interval exercise effectively improves cardiac function in patients with type 2 diabetes mellitus and diastolic dysfunction: a randomized controlled trial. J Am Coll Cardiol 2014;64:1758-60. [Crossref] [PubMed]
- Byrkjeland R, Njerve IU, Anderssen S, et al. Effects of exercise training on HbA1c and VO2peak in patients with type 2 diabetes and coronary artery disease: A randomised clinical trial. Diab Vasc Dis Res 2015;12:325-33. [Crossref] [PubMed]
- Lew JK, Pearson JT, Saw E, et al. Exercise Regulates MicroRNAs to Preserve Coronary and Cardiac Function in the Diabetic Heart. Circ Res 2020;127:1384-1400. [Crossref] [PubMed]
- Rawal S, Manning P, Katare R. Cardiovascular microRNAs: as modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc Diabetol 2014;13:44. [Crossref] [PubMed]
- Habibi P, Alihemmati A, Ahmadiasl N, et al. Exercise training attenuates diabetes-induced cardiac injury through increasing miR-133a and improving pro-apoptosis/anti-apoptosis balance in ovariectomized rats. Iran J Basic Med Sci 2020;23:79-85. [PubMed]
- Improta Caria AC, Nonaka CKV, et al. Exercise Training-Induced Changes in MicroRNAs: Beneficial Regulatory Effects in Hypertension, Type 2 Diabetes, and Obesity. Int J Mol Sci 2018;19:3608. [Crossref] [PubMed]
- Rawal S, Munasinghe PE, Shindikar A, et al. Down-regulation of proangiogenic microRNA-126 and microRNA-132 are early modulators of diabetic cardiac microangiopathy. Cardiovasc Res 2017;113:90-101. [Crossref] [PubMed]
- Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 2008;15:272-84. [Crossref] [PubMed]
- Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 2008;15:261-71. [Crossref] [PubMed]
- Jia Z, Wang J, Shi Q, et al. SOX6 and PDCD4 enhance cardiomyocyte apoptosis through LPS-induced miR-499 inhibition. Apoptosis 2016;21:174-83. [Crossref] [PubMed]
- Tijsen AJ, van der Made I, van den Hoogenhof MM, et al. The microRNA-15 family inhibits the TGFβ-pathway in the heart. Cardiovasc Res 2014;104:61-71. [Crossref] [PubMed]
- Ding D, Lawson KD, Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet 2016;388:1311-24. [Crossref] [PubMed]
- Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012;380:219-29. [Crossref] [PubMed]
- Crisafulli A, Pagliaro P. The COVID-19 Pandemic: A Challenge for the Cardiovascular Health. Curr Cardiol Rev 2020;16:vi-xi. [Crossref] [PubMed]
- Crisafulli A, Pagliaro P. Physical activity/inactivity and COVID-19. Eur J Prev Cardiol 2020;2047487320927597: Epub ahead of print. [Crossref]
Cite this article as: Femminò S, Pagliaro P. Exercise prevents cardiovascular dysfunctions in diabetes through miRNAs modulation. Non-coding RNA Investig 2021;5:1.


